A Galvanic Cell Generates A Potential Of 2.71 . M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. Solution the cell in figure 17.3 is galvanic, the spontaneous cell. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. E°cell = e°red + e°ox. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. The cell potential is created when the two dissimilar metals. what is the standard potential of the galvanic cell shown in figure 17.3? a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. E° cell for the given redox reaction is 2.71 v.
from www.numerade.com
E° cell for the given redox reaction is 2.71 v. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. E°cell = e°red + e°ox. Solution the cell in figure 17.3 is galvanic, the spontaneous cell. The cell potential is created when the two dissimilar metals. what is the standard potential of the galvanic cell shown in figure 17.3?
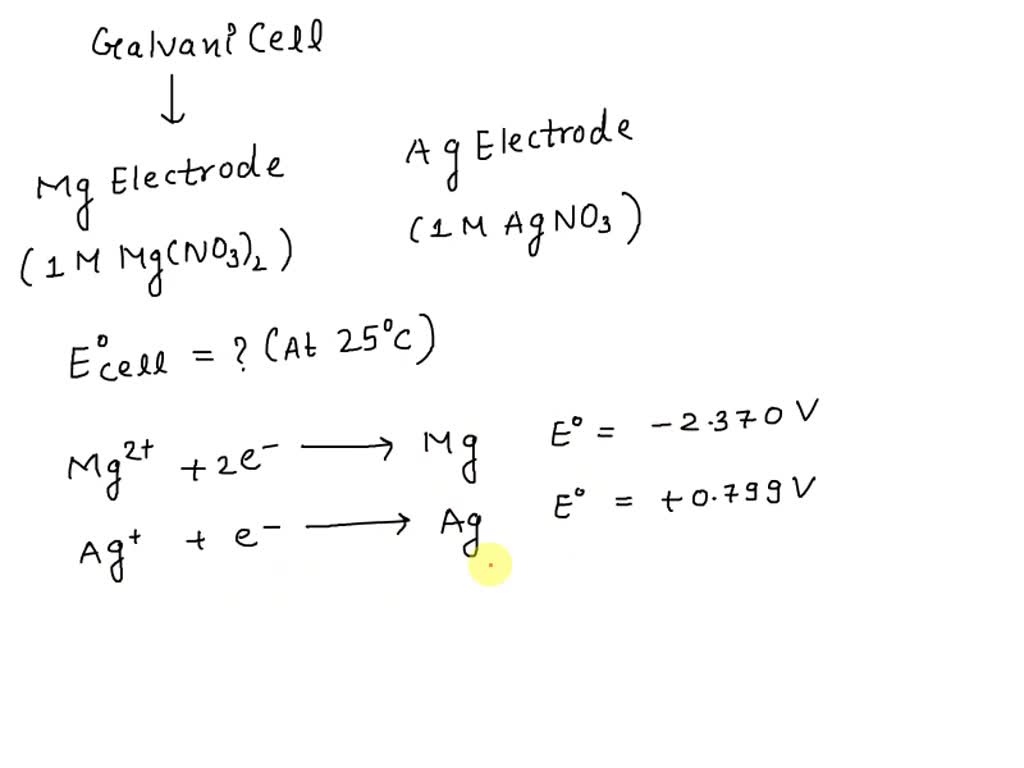
SOLVED A galvanic cell consists of a Mg electrode in 1 M Mg(NO3)2
A Galvanic Cell Generates A Potential Of 2.71 a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. what is the standard potential of the galvanic cell shown in figure 17.3? Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. E°cell = e°red + e°ox. a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. The cell potential is created when the two dissimilar metals. Solution the cell in figure 17.3 is galvanic, the spontaneous cell. E° cell for the given redox reaction is 2.71 v.
From www.numerade.com
Galvanic cell based on the above reaction constructed according to the A Galvanic Cell Generates A Potential Of 2.71 a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. the instant the circuit is completed, the voltmeter reads +0.46. A Galvanic Cell Generates A Potential Of 2.71.
From www.nanoscience.com
Electrochemistry Nanoscience Instruments A Galvanic Cell Generates A Potential Of 2.71 M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. E° cell for the given redox reaction is 2.71 v. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. what is the standard potential of the galvanic cell shown in figure 17.3? Solution the cell in figure 17.3 is galvanic,. A Galvanic Cell Generates A Potential Of 2.71.
From www.chegg.com
Solved A galvanic cell is prepared with tin and silver A Galvanic Cell Generates A Potential Of 2.71 a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. The cell potential is created when the two dissimilar metals. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. E°cell. A Galvanic Cell Generates A Potential Of 2.71.
From www.clutchprep.com
Galvanic Cell Chemistry Video Clutch Prep A Galvanic Cell Generates A Potential Of 2.71 The cell potential is created when the two dissimilar metals. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. what is the standard potential of the galvanic cell shown in figure 17.3? a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the.. A Galvanic Cell Generates A Potential Of 2.71.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 A Galvanic Cell Generates A Potential Of 2.71 E°cell = e°red + e°ox. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. The cell potential is created when the two dissimilar metals. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. M g(s)+cu2+. A Galvanic Cell Generates A Potential Of 2.71.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell Generates A Potential Of 2.71 The cell potential is created when the two dissimilar metals. a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. E° cell for the given redox reaction is 2.71 v. Cu?'. A Galvanic Cell Generates A Potential Of 2.71.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle A Galvanic Cell Generates A Potential Of 2.71 It is the electrode of an electrochemical cell that accepts electrons from the external circuit. The cell potential is created when the two dissimilar metals. E° cell for the given redox reaction is 2.71 v. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. E°cell = e°red + e°ox. Cu?' (aq). A Galvanic Cell Generates A Potential Of 2.71.
From solvedlib.com
Galvanic cell using the half reactions below What is … SolvedLib A Galvanic Cell Generates A Potential Of 2.71 the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. The cell potential is created when the two dissimilar metals. a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. E° cell for the given redox reaction is 2.71 v. Solution. A Galvanic Cell Generates A Potential Of 2.71.
From www.numerade.com
SOLVED A galvanic cell using AuAu and Zn2+Zn was set up at 299 K and A Galvanic Cell Generates A Potential Of 2.71 the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. what is the standard potential of the galvanic cell shown in figure 17.3? Solution the cell. A Galvanic Cell Generates A Potential Of 2.71.
From www.studypool.com
SOLUTION Galvanic cell their representation Studypool A Galvanic Cell Generates A Potential Of 2.71 what is the standard potential of the galvanic cell shown in figure 17.3? Solution the cell in figure 17.3 is galvanic, the spontaneous cell. The cell potential is created when the two dissimilar metals. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. Cu?' (aq) + mg (s) + cu (s) + mg?'. A Galvanic Cell Generates A Potential Of 2.71.
From www.youtube.com
Galvanic Cell Example Daniell Cell YouTube A Galvanic Cell Generates A Potential Of 2.71 Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. E° cell for the given redox reaction is 2.71 v. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. It is the electrode of an electrochemical cell that accepts electrons from. A Galvanic Cell Generates A Potential Of 2.71.
From www.numerade.com
SOLVED Using the image of the galvanic cell below, what is the A Galvanic Cell Generates A Potential Of 2.71 The cell potential is created when the two dissimilar metals. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. E°cell = e°red + e°ox. what is the standard potential of the galvanic cell shown in figure 17.3? a galvanic. A Galvanic Cell Generates A Potential Of 2.71.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell Generates A Potential Of 2.71 The cell potential is created when the two dissimilar metals. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. E°cell = e°red + e°ox. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. It is the electrode of an electrochemical. A Galvanic Cell Generates A Potential Of 2.71.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle A Galvanic Cell Generates A Potential Of 2.71 the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. Solution the cell in figure 17.3 is galvanic, the spontaneous cell. Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a galvanic cell generates a potential of +2.71 v when (cu) =. The cell potential. A Galvanic Cell Generates A Potential Of 2.71.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell Generates A Potential Of 2.71 The cell potential is created when the two dissimilar metals. a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. what is the standard potential of the galvanic cell shown in figure 17.3?. A Galvanic Cell Generates A Potential Of 2.71.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner A Galvanic Cell Generates A Potential Of 2.71 the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. Solution the cell in figure 17.3 is galvanic, the spontaneous cell. what is the standard potential of the galvanic cell shown in figure 17.3? Cu?' (aq) + mg (s) + cu (s) + mg?' (aq) e cell = +2.71 v a. A Galvanic Cell Generates A Potential Of 2.71.
From www.studypool.com
SOLUTION Chem ch3 galvanic cell pptx 2 Studypool A Galvanic Cell Generates A Potential Of 2.71 Solution the cell in figure 17.3 is galvanic, the spontaneous cell. It is the electrode of an electrochemical cell that accepts electrons from the external circuit. the instant the circuit is completed, the voltmeter reads +0.46 v, this is called the cell potential. what is the standard potential of the galvanic cell shown in figure 17.3? Cu?' (aq). A Galvanic Cell Generates A Potential Of 2.71.
From chem.eduinsightful.com
Galvanic Cells Decoded Igniting a Spark of Knowledge A Galvanic Cell Generates A Potential Of 2.71 The cell potential is created when the two dissimilar metals. M g(s)+cu2+ (0.01m) →m g2+ (0.001m)+cu(s) calculate ecell for the. E° cell for the given redox reaction is 2.71 v. a galvanic cell can also be used to measure the solubility product of a sparingly soluble substance and calculate the. It is the electrode of an electrochemical cell that. A Galvanic Cell Generates A Potential Of 2.71.